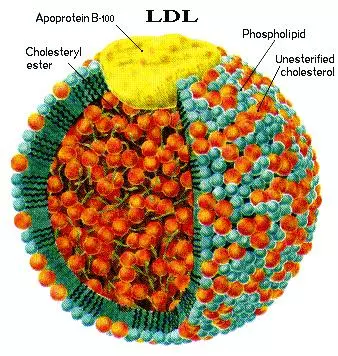
Apolipoprotein B (apoB) is the major protein component of chylomicrons, very low-density lipoproteins (VLDLs), intermediate-density lipoproteins (IDLs), lipoprotein(a) [Lp(a)], and low-density lipoproteins (LDLs), and constitutes 20-25% of the total weight of LDL. Overall, because of its much longer half-life (1.5-3 days), LDL comprises > 90% of the apoB-carrying lipoproteins.
ApoB exists in two forms, deriving from the same transcript of the APOB gene: apoB-100, the full-length and most abundant protein, is 4563 amino acids in length and is found in lipoproteins synthesized by the liver including LDL, IDL, and VLDL.1,2
ApoB-48, consisting of the N-terminal 2152 amino acids (48% of the molecular weight) of apoB-100, is produced by the small intestine and is found in chylomicrons.1,2 ApoB-48 is generated when a stop codon at residue 2153 is created by RNA editing, and lacks the C-terminal LDL receptor-binding domain of apoB-100.3
Theoretically, lipids derived from exogenous dietary sources are packaged in chylomicrons containing apoB-48, whereas endogenously produced lipids from the liver are packaged in particles containing apoB-100 (VLDL, IDL, LDL). The majority of absorbed cholesterol is of endogenous, not exogenous, origin.
However, it is important to note that chylomicrons rapidly exchange their core lipids with all endogenously produced particles via cholesteryl ester transfer protein (CETP); hence, all particles carry endogenous and exogenous lipids. A critical distinction between apoB-100 and apoB-48 is that the latter is not a ligand for the LDL receptor.
Because LDL particles contain one nontransferable molecule of apoB-100 and widely varying amounts of core cholesterol and triglycerides [a normally composed LDL has a ≥ 4:1 ratio of cholesteryl ester (CE) to triglycerides (TG)], apoB is a more reliable measurement of the relative number of LDL particles than is LDL-C.
Furthermore, the very long half-life of LDLs means that they comprise more than > 90% of the circulating apoB particles; apoB is hence simply a marker of LDL-P, which is an indicator of the number of potentially atherogenic particles. ApoB-100-containing particles are cleared through the hepatic LDL receptor, which also recognizes apoE (but not apoB-48) and is thus also called the apoB/apoE receptor.
The smaller or larger LDL particles, because of changes in the configuration of apoB, are not as readily cleared as normal-sized LDLs and their half-life is 3-5 days. Much of the risk related to small LDLs is their heavy contribution to total LDL-P. Furthermore, since small LDLs carry less cholesterol per particle, individuals with apparently normal LDL-C values can have discordantly high LDL-P or apoB levels and be at risk despite unremarkable LDL-C levels.
Since the major criteria determining apoB-containing particle entry into the arterial wall is particle number, the higher levels of plasma apoB may signify increased coronary disease risk even when LDL-C is not elevated. For comprehensive cardiovascular risk assessment, because of the numerical association of apoB (90% of which represents LDL-P), it is important to consider the apoB and LDL-P metrics and remember that neither apoB nor LDL-P is reported in a standard lipid panel. It is also important to recognize that apoB is not an accurate measure of VLDL or remnant lipoproteins—it is a primarily a biomarker of LDL-P.
Clinical Interpretation
It is well established that increased plasma concentrations of apoB-containing lipoproteins are associated with an increased risk of developing atherosclerotic disease.5-11 Case-control studies have found plasma apoB concentrations to be more discriminating than other plasma lipids in identifying patients with coronary heart disease (CHD).
The utility of apoB in determining CHD risk has been confirmed by prospective studies, although the extent to which apoB concentrations are better than serum lipids in predicting risk is variable (in general, discordance is highest in insulin resistant patients who typically have a higher core LDL-TG which leads to CE depleted LDLs).9
As mentioned above, apoB is a component of all atherogenic or potentially atherogenic particles, including chylomicrons and their remnants, VLDL and their remnants, IDL, LDL, and lipoprotein(a) [Lp(a)], and each particle contains one molecule of apoB. Therefore, apoB provides a direct measure of the number of atherogenic lipoprotein particles in the circulation. However, in normotriglyceridemic and hypertriglyceridemic patients the vast majority of total plasma apoB (over 90%) is associated with LDL, making apoB an effective surrogate for LDL particle concentration.
There is now a clear consensus that, when discordant with lipid measurements, apoB is more strongly predictive of CHD than LDL-C,12-14 and a recent consensus conference report from the American Diabetes Association (ADA) and the American College of Cardiology (ACC) recognizes the importance of measured (not calculated) apoB concentrations.9,13,15
Genetic ApoB deficiency
Abetalipoproteinemia is a fetal and pediatric autosomal recessive disorder in which no apoB is produced. The condition can cause malabsorption of food lipids, severely impaired trafficking of TG and fat soluble vitamins (especially vitamin E), and polyneuropathy.16
A milder condition, hypobetalipoproteinemia, associated with very low levels of LDL-C and apoB, is fully compatible with longevity due to the absence of coronary atherosclerosis.16 In patients with hyperbetalipoproteinemia, a disorder associated with increased risk of developing CHD, apoB may be concordant or discordant with LDL-C.17
ApoB and Cardiovascular disease
Virtually all lipoprotein disorders associated with atherosclerosis are characterized by increased serum apoB concentrations. ApoB mediates the uptake of LDL particles by liver and peripheral tissue via a specific interaction with the LDL receptor.
Familial hypercholesterolemia (FH) has multiple causes but may be due to a genetic defect in the LDL receptor that prevents the clearance of LDL particles from the circulation, or to a gain-of-function mutation in the proprotein convertase subtilisin/kexin type 9 (PCSK9) gene that leads to increased catabolism of the LDL receptors (again, reduced clearance leading to elevated plasma LDL-P).18,19
An increased number of plasma LDL particles is therefore a hallmark of FH. Familial defective apoB is a related FH disorder that arises from a genetic mutation in apoB that prevents its binding to the LDL receptor, resulting in a clinical phenotype similar to FH.20
In sporadic or polygenic hypercholesterolemia, which is likely due to any number of molecular defects increasing overproduction of LDL particles, heredity plays a smaller role. This is the situation for most individuals with elevated LDL-C, due to feedback suppression of LDLR gene expression by high dietary intake of cholesterol and saturated fats and/or other modifying genetic variants.21
Hypertriglyceridemia (HTG), associated with increased LDL particle number (and therefore apoB), may be the most common underlying cause of hyperbetalipoproteinemia, usually referred to as dyslipidemia or dyslipoproteinemia. A spectrum of common and rare lipid-associated genetic variants underlies this clinically heterogeneous condition.22
Hypertriglyceridemia without increased LDL particle concentration (familial HTG) is probably not atherogenic. The high TG in this instance are a consequence of very large VLDL particles with normal apoB levels. Pancreatitis but not atherosclerosis is typical.23
Type III dysbetalipoproteinemia, associated with the apoE2/E2 genotype, is a condition characterized by high TG and high cholesterol with unremarkable LDL-P levels (but excess remnant lipoproteins, predominantly small VLDL and IDL).24 The disorder is associated with peripheral and coronary artery disease; however, additional gene and environmental factors are necessary for expression of this hyperlipoproteinemia. One study noted that individuals with elevated Lp(a) levels also appeared to have elevated small, dense LDL particles.25
The most common and perhaps underdiagnosed lipoprotein disorder is familial combined hyperlipidemia (FCHL). FCHL was originally defined as a total cholesterol and/or triglycerides concentration > 95th percentile in probands with premature CHD and at least one affected first-degree relative.26 Subsequent research has identified an association of FCHL with an increase in total LDL-P (severe hyperbetalipoproteinemia) consisting of primarily small, dense LDL particles and determined that FCHL is most accurately diagnosed with a panel that includes measurement of apoB or LDL-P.27
The pathological defect in FCHL is multifactorial, including VLDL overproduction, delayed catabolism and decreased LDL clearance, as the small size of the LDL particles renders them unrecognizable to the LDL receptor. Because apoB is directly involved with defects of LDL synthesis or clearance, it is expected to play a central role in diagnosis and monitoring of these disorders.
AMORIS Study
Thompson and Danesh performed a meta-analysis of prospective studies of apoB and CHD.28 It is clear from this analysis that apoB is a significant predictor of cardiovascular disease with an overall relative risk of about 2.0 for the upper versus the lower tertile. Among the more compelling studies is the AMORIS Study because of the large number of subjects.29 More than 175,000 men and women over the age of 60 were followed up over a five-year period.
During this time, 864 men and 359 women suffered a fatal myocardial infarction (MI). After adjusting for age and traditional lipid risk factors, including LDL cholesterol, apoB remained a significant predictor of fatal MI with relative risks of 1.33 (confidence interval 1.17-1.51) and 1.53 (confidence interval 1.25-1.88) for a 1-SD increase in men and women, respectively. LDL-C was not a significant risk factor in women and was only modestly associated with MI in men.29,30
The Quebec Cardiovascular Study
This study followed 2,039 men, ages 45-76, for five years.31 ApoB was a strong, independent predictor of future cardiac events even after adjustment for age, smoking, systolic blood pressure, diabetes, and medication use. Interestingly, the investigators found a synergistic relationship between apoB and the total cholesterol/HDL cholesterol ratio (TC/HDL). When the TC/HDL ratio was low, an elevated apoB was associated with an increased risk of CHD (relative risk = 1.6), but when the TC/HDL ratio was high, an elevated apoB was associated with a markedly increased (and statistically significant) risk of CHD (relative risk = 2.6). A
13-year follow-up of the Quebec Cardiovascular Study participants also suggested a similar synergy between LDL cholesterol and apoB.32 Among the men with elevated LDL-C and a low concentration of apoB (< 128 mg/dL), relative risk for CHD was a modest 1.5, but when both LDL-C and apoB were elevated the relative risk was 2.2.
More Studies
A recent review of prospective studies comparing apoB and LDL-C as predictors of coronary artery disease and cardiovascular risk found that all but one of the 21 studies of apoB in primary prevention found a statistically significant association with CHD, even after adjustment for non-lipid risk factors.9
Of the 13 primary prevention studies that also provided data for LDL-C, only 9 reported a significant relationship between LDL-C and CHD in both men and women. Among the studies reporting both apoB and LDL-C, apoB was consistently the stronger risk factor.
The secondary prevention studies reported similar results. Baseline value of apoB was a significant predictor of recurrent cardiovascular events in the 4S, LIPID, THROMBO, and other studies.33-36 In contrast to LDL-P, neither apoB nor LDL-C was a significant predictor of recurrent events in the VAHIT Study; however, subjects were selected to have relatively low LDL-C concentrations.37
There is a wide variation in the reported relative risks for CHD in these epidemiologic studies, largely dependent on whether apoB is adjusted for other lipids and lipoproteins. Thus, the debate has become one of statistics rather than biological plausibility. The bigger issue is to not look at the patients with concordant LDL-P and LDL-C where both correlate well with outcomes, but to examine outcomes in patients where these measures are discordant.14
However, as the Quebec Cardiovascular Study and AMORIS have shown us, in large-scale studies with precise and standardized apoB measurement, apoB does appear to show statistically significant predictive effects even when traditional lipids and lipoproteins are covariates in the regression models. This is also evident in the Health Professionals Follow-up Study.38 When apoB and LDL-C were both simultaneously included in the model, relative risk for CHD was strongly associated with apoB while LDL-C and non-HDL-C were no longer statistically significant.
How to decrease ApoB
Statins are highly effective in reducing serum cholesterol through inhibition of HMG-CoA reductase, the rate- limiting enzyme in cholesterol synthesis. Subsequent depletion of hepatic cholesterol pools leads to the upregulation of LDL receptors and hence to increased clearance of LDL particles from the circulation.
Statins also reduce the production of both VLDL and subsequent LDL particles. However, the reduction in serum apoB or LDL-P concentration is not as dramatic as the reduction in LDL-C or non–HDL-C.39 As a result, patients treated to goal for LDL-C may not have achieved correspondingly low LDL particle concentrations (discordance), leaving them with potential residual risk.39-40
The Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPs) demonstrated that apoB at baseline and after one year on therapy was a strong predictor of future cardiovascular events, whereas LDL-C failed to reach significance (P=0.05 at baseline and on therapy).41 The LIPID study provided similar results.32 The reason is clear: LDL-related risk is not captured by LDL-C measurement alone.
Results from both primary and secondary statin trials suggest that on-therapy concentrations of apoB better predict future CHD events than does LDL-C.
References
- Olofsson SO, Boren J. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J Intern Med 2005;258(5):395-410.
- Knott TJ, Pease RJ, Powell LM, Wallis SC, Rall SC Jr, Innerarity TL, et al. Complete protein sequence and identification of structural domains of human apolipoprotein B. Nature (Lond) 1986;323:734–8.
- Teng B, Verp M, Salomon J, Davidson, NO. Apolipoprotein B messenger RNA editing is developmentally regulated and widely expressed in human tissues. J Biol Chem 1990;265(33):20616-620.
- Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 2006;26:847–70.
- Kwiterovich PO Jr, Coresh J, Smith HA, et al. Comparison of the plasma levels of apolipoproteins B and A-1, and other risk factors in men and women with premature coronary artery disease. Am J Cardiol 1992;69:1015-1021
- Stampfer MJ, Sacks FM, Salvini S, et al. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med 1991;325:373-381.
- Genest J, Marlin-Munley SS, McNamara JR, et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 1992;85:2025-2033
- Sniderman A, Shapiro S, Marpole D, et al. Association of Coronary Atherosclerosis With Hyperapobetalipoproteinemia [increased protein but normal cholesterol levels in human plasma low density (beta) lipoproteins]. Proc Natl Acad Sci USA 1980;77:604-608
- Contois JH, McConnell JP, Sethi AA, Csako G, Devaraj S, Hoefner DM, Warnick GR. Apolipoprotein B and Cardiovascular Disease Risk: Position Statement from the AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices. Clin Chem 2009;55(3):407–419
- Zambon A, Braun BG, Deeb SS, Brunzell JD. Genetics of apolipoprotein B and apolipoprotein AI and premature coronary artery disease. J Intern Med 2006;259:473–80.
- Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 2008;40:161–9.
- Sniderman AD, Williams K, Contois JH, Monroe HM, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes 2011;4(3):337-45.
- Davidson MH, Ballantyne CM, Jacobsen TA, et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: Advice from an expert panel of lipid specialists. J Clin Lipidol 2011;5(5):338-367.
- Otvos JD, Mora S, Shalaurova I, Greenland P, Macket RH, Goff DC. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol 2011;5(2):105-113.
- Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol 2008;15;51(15):1512-24.
- Burnett JR, Zhong S, Jiang ZG, Hooper AJ, Fisher EA, McLeod RS, et al. Missense mutations in APOB within the betaalpha1 domain of human APOB-100 result in impaired secretion of ApoB and ApoB-containing lipoproteins in familial hypobetalipoproteinemia. J Biol Chem 2007;282:24270–83.
- Tarugi P, Avema M. Hypobetalipoproteinemia: genetics, biochemistry, and clinical spectrum. Adv Clin Chem 2011;54:81-107.
- Nemati MH, Astaneh B. Optimal management of familial hypercholesterolemia: treatment and management strategies. Vasc Health Risk Manag 2010;6:1079-88.
- Liyanage KE, Burnett JR, Hooper AJ, van Bockxmeer FM. Familial hypercholesterolemia: epidemiology, Neolithic origins and modern geographic distribution. Crit Rev Clin Lab Sci 2011;48(1):1-18.
- Innerarity TL, Weisgraber KH, Arnold KS, Mahley RW, Krauss RM, Vega GL, Grundy SM. Familial defective apolipoprotein B-100: lowdensity lipoproteins with abnormal receptor binding. Proc Natl Acad Sci USA 1987;84:6919–23.
- Radar DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J Clin Invest 2003;111(12):1795-1803.
- Evans D, Aberle J, Beil FU. The relative importance of common and rare genetic variants in the development of hypertriglyceridemia. Expert Rev Cardiovasc Ther 2011;9(5):637-44
- Ewald N, hardt PD, Kloer HU. Severe hypertriglyceridemia and pancreatitis: presentation and management. Curr Opin Lipidol 2009;20(6):497-504.
- Schaefer JR. Unraveling hyperlipidemia type III (dysbetalipoproteinemia), slowly. Eur J Hum Genet 2009;17:541-542.
- Moon JY, Kwon HM, Kwon SW, et al. Lipoprotein(a) and LDL particle size are related to the severity of coronary artery disease. Cardiology 2007;108(4):282-9.
- Wierzbicki AS, Graham CA, Young IS, Nicholls DP. Familial combined hyperlipidemia: under-defined and under-diagnosed? Curr Vasc Pharmacol 2008;6(1):13-22.
- Veerkamp MJ, de Graaf J, Hendriks JCM, Demacker PNM, Stalenhoef AFH. Nomogram to diagnose familial combined hyperlipidemia on the basis of results of a 5-year follow-up study. Circulation 2004;109:2980–5.
- Thompson A, Danesh J. Association between apolipoprotein B, apolipoprotein AI, the apolipoprotein B/AI ratio and coronary heart disease: a literature-based meta-analysis of prospective studies. J Intern Med 2006;259:481–92.
- Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-1, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet 2001;358:2026–33.
- McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, et al., for the INTERHEART study investigators Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet 2008;372:224–33.
- Lamarche B, Moorjani S, Lupien PJ, et al. Apolipoprotein A-1 and B levels and the risk of ischemic heart disease during a 5 year followup of men in the Quebec Cardiovascular Study. Circulation 1996;94:273-8.
- St-Pierre A, Cantin B, Dagenais GR, et al. Low-density lipoprotein subfractions and the long term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol 2005;25:553–9.
- Simes RJ, Marschner IC, Hunt D, Colquhoun D, Sullivan D, Stewart RAH. Relationship between lipid levels and clinical outcomes in the long-term intervention with pravastatin in the ischemic disease (LIPID) trial: to what extent is the reduction in coronary events with pravastatin explained by on-study lipid levels? Circulation 2002;105:1162–9.
- Benn M, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A. Improving prediction of ischemic cardiovascular disease in the general population using apolipoprotein B: the Copenhagen City Heart Study. Arterioscler Thromb Vasc Biol 2007;27:661-70.
- Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998;97:1453–60.
- van Lennep JE, Westerveld HT, van Lennep HW, Zwinderman AH, Erkelens DW, van der Wall EE. Apolipoprotein concentrations during treatment and recurrent coronary artery disease events. Arterioscler Thromb Vasc Biol 2000;20:2408–13.
- Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 2006;113:1556–63.
- Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation 2005; 112:3375–83.
- Sniderman AD. Differential response of cholesterol and particle measures of atherogenic lipoproteins to LDL-lowering therapy: implications for clinical practice. J Clin Lipidol 2008;2:36–42.
- Walldius G, Jungner I. Apolipoprotein B and apolipoprotein AI: risk indicators of coronary heart disease and targets for lipid-modifying therapy. J Intern Med 2004;255:188–205.
- Gotto AM, Whitney E, Stein EA, Shapiro DR, Clearfield M, Weis S. Relation between baseline and on-treatment lipid parameters and first acute major coronary events in the Air force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/ TexCAPS). Circulation 2000;101:477–84.