We measure Myeloperoxidase (MPO) as part of our Functional Medicine health evaluation. It is a part of our “full panel” lab assessment. High levels of myeloperoxidase correlate with an increased risk of cardiovascular disease.
Myeloperoxidase (MPO) is an enzyme derived from white blood cells (Leukocytes). It plays a role in the inflammatory response and helps to kill invading pathogens by generating reactive oxidants. Myeloperoxidase (MPO) is released from macrophages when the arterial wall is inflamed or damaged.
The purpose of the immune system is to protect us from the invading organisms and pathogens. Myeloperoxidase is a critical part of this system.
However, cardiovascular disease is not really a disease. It is a process. It is a condition where the body is responding to one of over 400 ‘insults’ which are threatening (or at least identified as such) our bodies. These insults are toxic and cause the immune system to fire up. Myeloperoxidase is both a marker that the immune system is activated and an actual contributor to the cardiovascular disease process.
These appropriate responses become exaggerated and chronic resulting in activation of the ‘3 finite responses’ and subsequent cardiovascular disease.
Myeloperoxidase (MPO) has several potential effects that increase the development of plaque such as:
- decreases Nitric Oxide (NO)
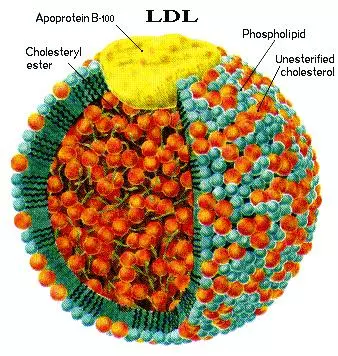
- oxidizes LDL forming oxLDL
- oxidizes HDL making it dysfunctional.
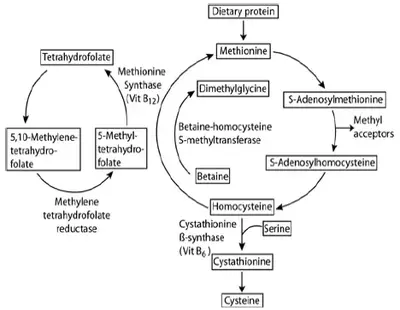
Nitric oxide (NO) produced by endothelial nitric oxide synthase (NOS) is a powerful vasodilator and as such plays a critical role in the regulation of vascular tone. Additionally, NO suppresses binding of circulating cells to the endothelium and inhibits proliferation of smooth-muscle cells in the vascular wall. Taken together, these findings indicate that NO is a critical element in vascular homeostasis, and consequently insufficient production and/or increased scavenging of NO may impair vascular function and accelerate atherosclerosis.
There are strong indications that MPO, by several mechanisms, may reduce the bioavailability of NO.
- NO serves as a substrate for peroxidases, and MPO may thus serve as a catalytic sink for NO.6,7
- Scavenging of NO by MPO-derived reactive substances may further reduce the bioavailability of NO.
- Hypochlorous acid can react with nitrogen atoms of the NOS substrate arginine to produce chlorinated arginine species that are inhibitors of all isoforms of NOS and have been shown to impair endothelium-dependent relaxation of rat aortic rings.8
- It has been demonstrated that hypochlorous acid is a potent inducer of uncoupling of endothelial NOS, thereby turning NOS into a superoxide-producing enzyme.9
Although the relative impact of these mechanisms is currently unknown, it is clear that MPO, by catalytic as well as non-catalytic processes, depletes NO in the vascular wall.
Interpretation
The clinical utility of MPO testing is well documented in more than 100 manuscripts published peer-reviewed journals, including the New England Journal of Medicine, the Journal of the American College of Cardiology, the American Journal of Cardiology, Circulation, JAMA, and others.
Specifically, MPO testing has proven clinically useful in primary prevention, secondary prevention, and heart failure settings, allowing physicians to appropriately classify various types of patients into risk categories in order to provide optimal lifestyle modifications or implement treatment strategies.
Primary Prevention Studies
Various studies have documented that increasing MPO levels predict increasing risk in various cohorts of individuals. In primary prevention studies, elevated MPO levels are a strong predictor of endothelial dysfunction in healthy individuals independent of Framingham risk score, prevalent cardiovascular disease, cardiovascular medications, and CRP.10
EPIC-Norfolk Prospective Population Study
The EPIC-Norfolk Prospective Population study demonstrated that elevated MPO levels are associated with the future risk of developing coronary artery disease (CAD) in healthy individuals even beyond traditional biomarkers, including LDL, HDL, and CRP.11
EISNER Study
The EISNER study demonstrated that elevated MPO levels are associated with increased risk of CVD events (MI, stroke, or death) in healthy individuals.12
Cardiovascular health Study
The Cardiovascular Health study demonstrated that elevated MPO levels are associated with the development of heart failure in healthy individuals.13
Secondary Prevention Studies
In secondary prevention studies, a single baseline MPO measurement independently predicts the risk for major adverse cardiac events in individuals with chest pain, but persistently negative for troponin T.14
The ADEPT study demonstrated that elevated MPO levels are associated with an increased likelihood of more advanced heart failure and predict long-term clinical outcomes in individuals with heart failure.
Treatment
Short-term rosuvastatin reduces inflammation in individuals with heart failure by reducing MPO levels. Animal studies have also demonstrated that acetaminophen has considerable potential as a therapeutic inhibitor of MPO-mediated tissue damage,16 and niacin inhibits vascular inflammation and prevents endothelial dysfunction by inhibiting MPO accumulation.17
References
- Klebanoff SJ, Waltersdorph AM, Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol 1984;105:399-403.
- Tavora FR, Ripple M, Li L, et al. Monocytes and neutrophils expressing myeloperoxidase occur in fibrous caps and thrombi in unstable coronary plaques. BMC Cardiovasc Disord 2009;9:27.
- Eiserich JP, Baldus S, Brennan ML, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 2002;296(5577):2391-4.
- Podrez EA, Schmitt D, Hoff HF, et al. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest 1999;103(11):1547-60.
- Zheng L, Nukuna B, Brennan ML, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest 2004;114(4):529-41.
- Baldus S, Heitzer T, Eiserich JP, et al. Myeloperoxidase enhances nitric oxide catabolism during myocardial ischemia and reperfusion. Free Radic Biol Med 2004;37(6):902-11.
- Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem 2000;275(48):37524-32.
- Yang J, Ji R, Cheng Y, et al. L-arginine chlorination results in the formation of a nonselective nitric-oxide synthase inhibitor. J Pharmacol Exp Ther 2006;318(3):1044-9.
- Xu J, Xie Z, Reece R, et al. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol 2006;26(12):2688-95.
- Vita JA, Brennan ML, Gokce N, et al. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 2004;110(9):1134-9.
- Meuwese MC, Stroes ES, Hazen SL, et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol 2007;50(2):159-65.
- Wong ND, Gransar H, Narula J, et al. Myeloperoxidase, subclinical atherosclerosis, and cardiovascular disease events. JACC Cardiovasc Imaging 2009;2(9):1093-9.
- Tang WH, Katz R, Brennan ML, et al. Usefulness of myeloperoxidase levels in healthy elderly subjects to predict risk of developing heart failure. Am J Cardiol 2009;103(9):1269-74.
- Brennan ML, Penn MS, Van Lente F, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 2003;349(17):1595-604.
- Tang WH, Tong W, Troughton RW, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol 2007;49(24):2364-70.
- Koelsch M, Mallak R, Graham GG, et al. Acetaminophen (paracetamol) inhibits myeloperoxidase-catalyzed oxidant production and biological damage at therapeutically achievable concentrations. Biochem Pharmacol 2010;79(8):1156-64.
- Wu BJ, Yan L, Charlton F, et al. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler Thromb Vasc Biol 2010;30(5):968-75.