FOXO4-DRI: A Deep Dive Into the Senolytic Peptide Rebooting Cellular Ageing
Introduction
In the field of longevity medicine and anti-ageing research, the focus has shifted from merely managing the consequences of ageing toward confronting the underlying cellular processes themselves. One of the most intriguing compounds in this arena is the synthetic peptide FOXO4-DRI. By interrupting a key protein–protein interaction that allows senescent cells to persist, FOXO4-DRI offers the potential to selectively eliminate those cells and thus improve tissue function, reduce inflammation, and restore aspects of youthful physiology.
In this comprehensive article we’ll explore what FOXO4-DRI is, how it works, the current pre-clinical evidence, its promise and limitations, comparisons with other senolytic approaches, safety and regulatory considerations, and what the future might hold for integrating this novel peptide into a clinical strategy for healthier ageing. As always, this is a research-level exploration and not a clinical protocol — any human use must await rigorous trials and regulatory approval.



What is FOXO4-DRI?
The peptide FOXO4-DRI stands for FOXO4 D-retro-inverso peptide. It is a synthetic, engineered peptide derived from the transcription factor FOXO4. What makes it special is that it is designed to disrupt the interaction between FOXO4 and the tumor-suppressor protein p53 within senescent cells, thereby triggering programmed cell death (apoptosis) of those cells. (Regentherapy)
In more detail:
-
FOXO4 is a member of the FOXO (Forkhead box O) transcription factor family, which plays major roles in stress response, metabolism, cellular longevity and homeostasis. (Wikipedia)
-
In senescent cells (cells that have permanently exited the cell‐cycle, accumulate damage, and secrete inflammatory factors), FOXO4 expression goes up and helps bind p53 in the nucleus, preventing p53 from driving those cells into apoptosis. (Peptide Sciences)
-
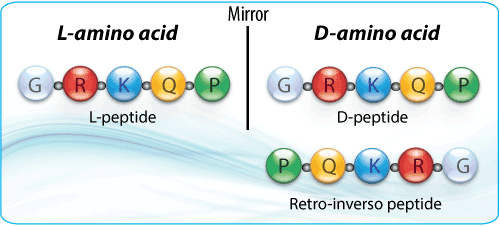
FOXO4-DRI is built using a D-retro-inverso peptide topology (using D‐amino acids, reversed sequence) which makes it highly stable and resistant to proteolysis. (Peptide Gurus)
-
By mimicking the part of FOXO4 that binds p53, FOXO4-DRI competes for that interaction, frees p53, causes its nuclear exclusion and triggers apoptosis in the senescent cell, thereby selectively reducing senescent cell burden. (Peptide Sciences)
The result: a novel senolytic (literally “senescent cell-killing”) peptide, offering a more targeted alternative to older senolytics that rely on broad stress induction or BCL2-inhibition.
Why target senescent cells? The senescence problem
To appreciate the significance of FOXO4-DRI, it helps to understand cellular senescence and why it matters in ageing and disease.
What are senescent cells?
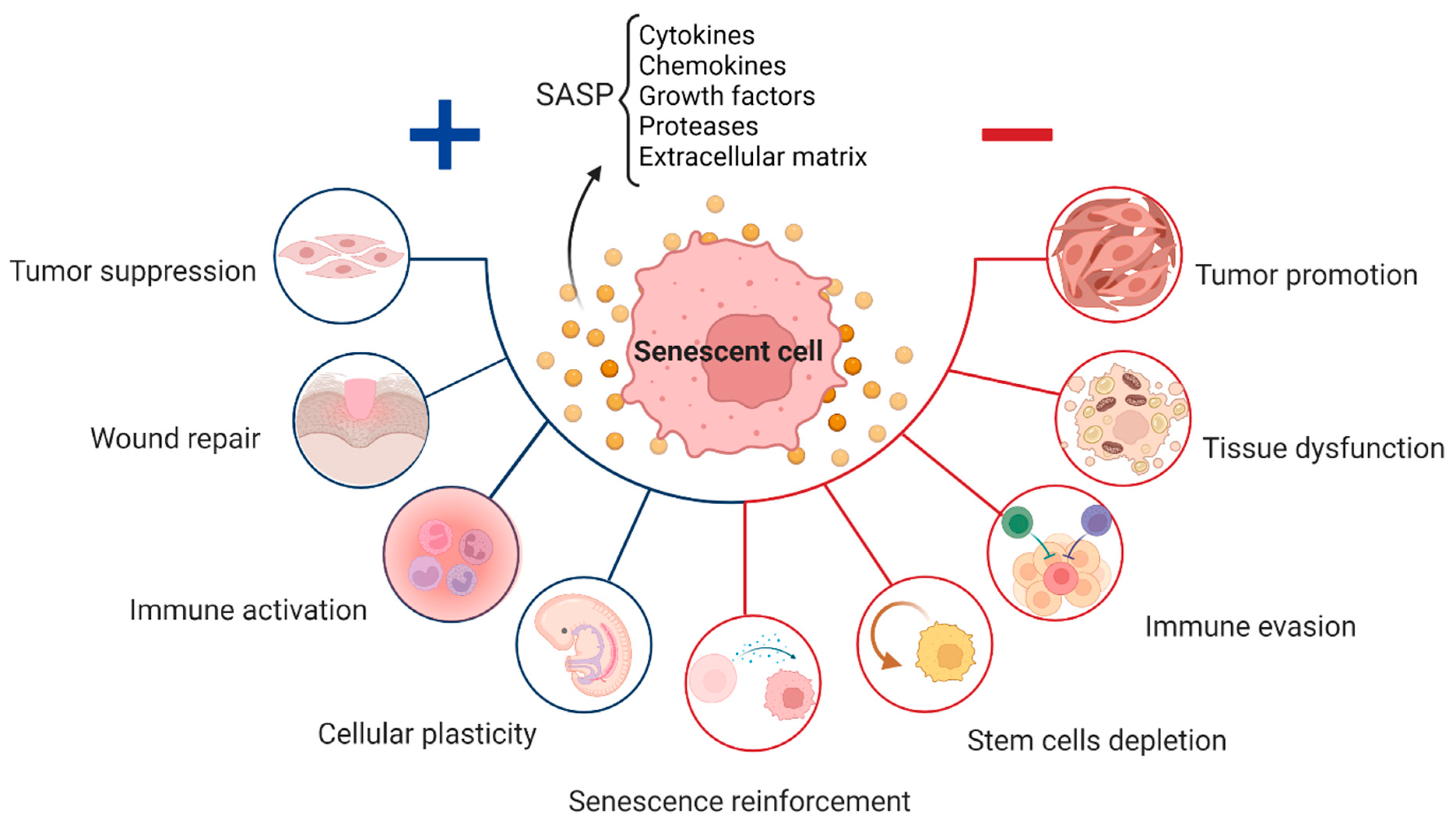
Senescence is a state in which a cell permanently exits the cell‐cycle (no longer divides) in response to stress such as DNA damage, telomere shortening, oncogene activation, oxidative stress, or replicative exhaustion. These cells remain metabolically active, adopt a distinct phenotype (including chromatin changes), and critically secrete a variety of pro-inflammatory cytokines, proteases, growth-factors and extracellular-matrix modifying signals. This secretory phenotype is known as the senescence-associated secretory phenotype (SASP). (Peptide Sciences)
While senescence is protective in acute contexts (e.g., preventing malignant transformation, wound repair), the accumulation of senescent cells over time is detrimental:
-
They occupy tissue space and resources, impairing progenitor cell function. (Biotech Peptides)
-
The SASP incites local chronic inflammation, tissue dysfunction, matrix breakdown, fibrosis, and impairs regeneration. (River Peptides)
-
When immune clearance of senescent cells declines with age, their accumulation accelerates organ dysfunction, frailty, age-related disease (cardiovascular disease, osteoarthritis, kidney decline, endocrine decline, etc). (Regentherapy)
Hence, removing senescent cells is a promising strategy for “health-span” extension (improving the period of life spent in good health) and possibly delayed onset of age-related diseases.
The senolytic approach
Senolytics are therapies designed to selectively eliminate senescent cells. Some approaches include: BCL-2/BCL-xL inhibitors (e.g., navitoclax), tyrosine kinase inhibitors, metabolic stressors, or even immune-based clearance. However many of these have off-target toxicity, low specificity, or are in early stages.
FOXO4-DRI distinguishes itself by offering a molecularly specific intervention: disrupting the FOXO4–p53 interaction, which appears to be a survival mechanism especially enriched in senescent cells. (River Peptides)
Thus FOXO4-DRI belongs to the next generation of senolytic tools with improved selectivity and lower “collateral damage” risk (at least in preclinical models).
Mechanism of Action of FOXO4-DRI
Let’s dig deeper into how FOXO4-DRI works — the biology, molecular targets, and how it produces its effects.
The FOXO4–p53 axis in senescence
-
p53 is a major tumour suppressor and master regulator of cellular stress responses: DNA damage, oxidative stress, oncogene activation. It can trigger cell‐cycle arrest, repair or apoptosis.
-
In senescent cells, p53 is often sequestered in the nucleus by FOXO4. This binding helps keep senescent cells alive, despite accumulating damage and being no longer useful. (In essence, FOXO4 “holds” p53 so it cannot execute the death program). (Peptide Sciences)
-
FOXO4 expression is increased in senescent cells, and interfering with FOXO4 via shRNA or peptide competition leads to apoptosis of those cells. (Peptide Sciences)
How FOXO4-DRI intervenes
-
FOXO4-DRI is constructed as a D-retro-inverso version of the FOXO4 segment that binds p53. Because of its D-amino acid configuration and reversed sequence, it is more stable in vivo and resists enzymatic degradation. (Xcel Peptides)
-
It includes a cell-penetrating segment that allows it to enter cells and reach the nucleus. (Particle Peptides)
-
Once inside, FOXO4-DRI competes with endogenous FOXO4 for p53 binding. It displaces p53 from the FOXO4 complex, leading to nuclear exclusion of p53 and activation of the intrinsic apoptosis pathway in the senescent cell. Healthy, dividing cells—which rely less on the FOXO4–p53 survival mechanism—are spared. (Peptide Sciences)
Resulting effects
-
Selective apoptosis of senescent cells ⇒ reduced senescent cell burden.
-
Lower SASP secretion (less inflammatory cytokines, proteases) ⇒ improved tissue microenvironment. (Peptide Sciences)
-
Improved tissue homeostasis: Possibly improved regeneration, improved organ function, improved resilience to stress. For example: better fur density, better kidney function, improved physical activity in aged mice. (Peptide Sciences)



Preclinical Evidence: What Has Research Shown So Far?
Given that FOXO4-DRI remains in the preclinical arena (i.e., animal models, in-vitro cell culture), let’s review key studies and what they suggest.
Landmark 2017 Cell paper
One of the foundational studies (by Baar et al.) was titled “Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging.” This work demonstrated:
-
In mice that were aged or given chemotherapy (accelerated ageing), treatment with FOXO4-DRI improved physical performance, fur quality, and renal function. (Peptide Sciences)
-
Importantly: selective removal of senescent cells without significant impact on non-senescent cells. This was a proof-of-principle that senescent cell clearance could restore organismal homeostasis.
Additional models: cartilage, endocrine, kidney
-
In cartilage/chondrocyte culture models: FOXO4-DRI reduced the number of senescent chondrocytes significantly, while healthy chondrocytes remained intact. This is relevant for osteoarthritis and joint regeneration research. (Particle Peptides)
-
Endocrine function: In aged male mice, FOXO4-DRI cleared senescent Leydig cells (testicular) and increased testosterone levels. This suggests endocrine tissue rejuvenation may be feasible. (Particle Peptides)
-
Kidney/renal function: Studies showed improving tubular cell markers, urea/creatinine metrics, and reducing SASP cytokines in kidney tissue. (Peptide Sciences)
Comparative advantage and specificity
Researchers note that many senolytics suffer from off-target cytotoxicity (i.e., they kill healthy cells too). The selectivity of FOXO4-DRI appears to be a major advantage:
“FOXO4-DRI peptide is particularly compelling due to its ability to disrupt the FOXO4-p53 interaction, a critical regulator of cellular senescence. … The precision of this mechanism is a key advantage, as it minimises collateral damage to surrounding tissues.” (Peptide Gurus)
Summary of findings
In summary:
-
FOXO4-DRI has been shown to reduce senescent cell number in animal models and cell culture.
-
It improves metrics of tissue function, regeneration and homeostasis in aged or stress-accelerated models.
-
It appears more selective and possibly safer than older senolytic approaches.
-
But: It remains experimental. There are no large human trials published yet. The safety, dosage, long-term effects aren’t fully defined.
Potential Applications & Benefits
Given the mechanism and preclinical evidence, what are the potential applications of FOXO4-DRI? Here are some of the most discussed.
Healthy ageing and longevity support
By reducing senescent cell burden, FOXO4-DRI may delay tissue decline, reduce chronic inflammation, improve organ resilience, and thus extend “health-span” (time lived in good health). The idea is not just more years, but better years. (Particle Peptides)
Regenerative medicine
Senescent cells impair tissue regeneration (by occupying space, secreting SASP, promoting fibrosis). Eliminating them may create a more favourable environment for stem/progenitor cells, enhance wound healing, cartilage repair, organ repair. The cartilage/chondrocyte data supports this. (Particle Peptides)
Endocrine rejuvenation
As noted, research showing improved testosterone production in aged mice suggests application in hormone decline, endocrine ageing syndromes (menopause/andropause), though this is very early. (Particle Peptides)
Chronic disease and degenerative conditions
Senescent cells play a role in osteoarthritis, kidney disease, cardiovascular disease, lung fibrosis, perhaps neurodegeneration (via chronic SASP). Thus FOXO4-DRI may be investigated for those conditions. For example, a blog noted applications for lung fibrosis (COPD/emphysema) via senescent fibroblasts. (Peptide Sciences)
Oncology & adjunctive therapy
Interestingly, since senescent cells often accumulate in tissues post-chemotherapy and may contribute to relapse or fibrosis, FOXO4-DRI might serve as adjuncts in oncology (preclinical) by clearing therapy-induced senescent cells. (Innopeptides)
Key Limitations, Risks & Unanswered Questions
As promising as FOXO4-DRI is, there are important caveats. No compound is magic. A balanced view is essential.
Human data lacking
-
To date, most (if not all) data is preclinical (rodents, cell culture).
-
No finalized large-scale human clinical trials are published that demonstrate safety and efficacy in humans.
-
Thus dosage, pharmacokinetics, long-term safety, off-target effects in humans remain unknown.
p53 tumour-suppressor caution
-
Because FOXO4-DRI intervenes in the p53 pathway, which is critical for tumour suppression, there is theoretical concern that interfering with p53 dynamics might carry oncogenic risk. (Although the peptide aims to free p53 to kill senescent cells, not inhibit p53).
-
Some researchers highlight this caution explicitly. (River Peptides)
Selectivity and “healthy cell” safety
-
Although evidence suggests selectivity, senescent cells are heterogeneous (not all rely equally on FOXO4–p53). Some healthy or quiescent cells may share features; the risk of unintended apoptosis remains.
-
The tissue distribution, biodistribution, immunogenicity of D-retro‐inverso peptides in humans need evaluation.
Long-term effects of senescent cell clearance
-
Some senescent cells play beneficial roles (wound healing, development, tumour suppression). Blanket clearance may have unintended consequences.
-
The timing, frequency, and extent of senescent cell removal remain open questions (for example, “when is it best to clear them?” “What is the rebound or regenerative consequence?”).
Regulatory & legal status
-
FOXO4-DRI is not an FDA-approved therapy. According to some peptide-wiki summaries: “Available exclusively from authorized research suppliers. Not approved or evaluated by the FDA for human use.” (Regentherapy)
-
Thus any use in humans—outside a formal clinical trial—is off-label and subject to regulatory risks (depending on jurisdiction).
Cost, manufacturing, reproducibility
-
Synthesising D-retro-inverso peptides with high purity is non-trivial. Batch reproducibility, stability, quality control matter. Some peptide-supplier blogs emphasise >98 % purity is required. (Innopeptides)
-
Long-term storage, delivery (penetration into tissues, safety of cell-penetrating segments) are still being worked out.
Comparison to Other Senolytic Therapies
To contextualise FOXO4-DRI, let’s compare it to other senolytic approaches or peptides you may be aware of (helpful given your clinic’s peptide focus).
| Approach | Mechanism | Pros | Cons |
|---|---|---|---|
| BCL-2/BCL-xL inhibitors (eg navitoclax) | Inhibit anti-apoptotic proteins in senescent cells | Established mechanistic target; some human data | Significant toxicity (thrombocytopenia, neutropenia) |
| Dasatinib + Quercetin | Tyrosine kinase inhibitor + flavonoid – induce apoptosis in senescent cells | Some human pilot studies in fibrosis/osteoarthritis | Less selective, off-target effects, variable results |
| FOXO4-DRI | Disrupt FOXO4–p53 binding; selective apoptosis in senescent cells | High specificity, promising regeneration data | Early stage, no human data, p53 safety concerns |
| Other peptides/regulators (e.g., humanin, MOTS-c) | Mitochondrial stress regulators, anti-inflammatory peptides | Broad metabolic effects, lower risk | Less specific for senescence, more indirect effect |
So, in summary: FOXO4-DRI appears to represent the “next-generation” senolytic, offering improved specificity, but it is still earlier stage than many small-molecule senolytics or repurposed drug combinations.
Practical Considerations for Research & Clinical Context
Given your clinic’s focus on peptides, hormone replacement, body composition, DEXA scanning and regenerative medicine, how might FOXO4-DRI fit into the broader strategy—and what to watch out for?
Integration in a clinic specialising in hormone replacement, body composition & regenerative medicine
-
Tissue regeneration and body composition improvements (e.g., better muscle regeneration, improved organ function, less fibrosis) may be a compelling angle. FOXO4-DRI may support rejuvenation of endocrine tissue, muscle, and visceral declines associated with ageing.
-
Given your DEXA scanner usage (body composition, visceral fat tracking), elimination of senescent cells might theoretically improve visceral fat inflammation, adipose tissue health, and metabolic function (though data is indirect).
-
For patients already on hormone replacement therapy (HRT), the notion of reducing senescent cell load may complement hormone optimisation by reducing the “age burden” on the tissues.
Protocol caution
-
Although your clinic dispenses peptides, it’s crucial to emphasise that FOXO4-DRI remains research only. Clearly disclaim human use unless within an approved clinical trial.
-
Before human translation, essential parameters to define: optimal dosing, infusion/administration route, frequency, biomarkers to monitor (senescent cell burden, inflammatory cytokines, organ function), long-term follow-up for adverse outcomes (especially tumour risk).
-
Ideally incorporate rigorous baseline and follow-up assessments: DEXA, body composition, visceral fat, renal function, endocrine panels, inflammatory biomarkers (IL-6, TNF-α, SASP markers if available).
-
Ensure clean sourcing of peptides with HPLC purity (>98 %), confirm stability, and safe handling protocols (sterile, GMP or research-grade). The supplier blogs emphasise < 2 % impurities. (Innopeptides)
Monitoring strategies
-
Pre-treatment: full panel including CBC, kidney/liver, endocrine, inflammatory cytokines, baseline DEXA, body comp, visceral fat.
-
Follow-up: serial DEXA/body comp at 3-6-12 months, inflammatory biomarkers, kidney/liver panels, hormone levels, any adverse events.
-
Optional research biomarkers: senescence markers (p16^INK4a, SA-β-gal), SASP cytokine profile (IL-6, IL-1β, MMPs) if lab resources permit.
Ethical/regulatory aspects
-
Given the novelty, informed consent must emphasise “off-label / investigational” status.
-
Documentation (protocol, IRB/ethics review if applicable, data collection plan) is strongly recommended.
-
Insurance billing may be difficult if labelled as “research only”. Disclose to patients appropriately.
Safety & Risk Mitigation
Here are key safety issues and how to mitigate them in a responsible setting.
Tumor risk via p53 pathway interference
Mitigate risk by:
-
Careful patient selection: exclude patients with pre-existing active malignancy or high tumour risk (e.g., known p53 mutations, familial cancer syndromes).
-
Comprehensive baseline cancer screening appropriate for age and risk (PSA, colonoscopy, mammography, skin exam).
-
Ongoing monitoring of tumour markers or imaging if indicated.
-
Conservative dosing initially (in research context) and close follow-up for signs of malignant transformation.
Immunogenicity / peptide safety
-
Verify peptide purity, endotoxin levels, proper storage and handling.
-
Monitor for allergic reactions, injection site reactions (if administered SubQ/IV).
-
Monitor liver enzymes, kidney function, complete blood count for any unexpected cytopenias or organ stress.
Off-target senescent cell clearance
-
Although senescent cell clearance is beneficial, some senescent cells are beneficial (e.g., during wound healing). Thus, timing matters — avoid initiating during active wound repair phase.
-
Monitor for impaired wound healing or tissue regeneration anomalies.
-
Consider pausing other regenerative therapies concurrently to avoid over-removal of senescent scaffolding cells.
Future Outlook & Research Directions
Where might FOXO4-DRI go from here? The trajectory suggests exciting possibilities, but also key research gaps.
Next steps in clinical translation
-
First-in-human safety trials (phase I) are essential: to assess dose tolerability, pharmacokinetics, biodistribution, peptide half-life, clearance, immunogenicity.
-
Biomarker studies: establishing senescent cell burden diagnostic (e.g., imaging, blood markers) to monitor effect and guide dosing.
-
Controlled trials in age-related diseases: e.g., osteoarthritis, chronic kidney disease, endocrine decline, frailty syndrome, possibly cardiovascular ageing.
-
Long-term follow-up for tumour incidence, organ function, regeneration outcomes.
Potential synergies
-
Combining FOXO4-DRI with regenerative therapies: e.g., stem cell therapies, hormone replacement, peptides promoting regeneration (such as GHK-Cu, MOTS-c) — clearing senescent cells may “clear the field” for regeneration. Some blogs suggest synergy (e.g., FOXO4-DRI + MOTS-c). (Peptide Sciences)
-
Use in post-treatment settings: e.g., after chemo/radiation when senescent cells accumulate and impair recovery, or in surgical recovery settings.
-
Integration with metabolic therapies: since senescent adipose tissue, liver steatosis and visceral fat contribute to metabolic decline, FOXO4-DRI might improve metabolic resilience.
Market & regulatory implications
-
If human translation succeeds, FOXO4-DRI or analogues may form the backbone of “senotherapy” clinics alongside peptides, hormones and regenerative medicine.
-
Ethical considerations: equity of access to life-extension therapies, off-label usage, “anti-ageing” claims and regulatory scrutiny.
Key unanswered research questions
-
What is the optimal dosing regimen: how often to administer, how long does effect last, when to retreat?
-
What biomarkers reliably measure senescent cell clearance in humans?
-
Which tissues respond best (muscle, endocrine, kidney, brain)?
-
What are the long-term consequences of widespread senescent cell clearance? Might some functions be impaired?
-
Patient population stratification: which patients will benefit most (frail older, endocrine decline, metabolic syndrome)?
-
Safety in humans: tumor risk, immunogenicity, organ side-effects.
Conclusion
The advent of peptides like FOXO4-DRI marks a paradigm shift in how we think about ageing—not merely as a linear time process we endure, but as a cellular process we may influence. By targeting the stubborn, pro-inflammatory senescent cells that accumulate with age, FOXO4-DRI offers the possibility of clearing the cellular “garbage” that impedes regeneration, contributes to chronic disease and ageing decline.
While the clinical translation is still in its early days, the mechanistic rationale is compelling, and the preclinical evidence is impressive. For clinics focused on hormone replacement, body composition, and regenerative medicine (like yours), FOXO4-DRI represents an exciting frontier to watch—and possibly incorporate into a future-rigorous, research-informed protocol.
As always, patient education, informed consent, rigorous monitoring and a conservative, evidence-based approach are essential. Ageing is complex, but with the right tools and strategies, we may be heading into a new era of active, healthy longevity.
References
-
Baar M.P. et al., “Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging.” Cell, 2017. (Peptide Sciences)
-
PeptideGurus, “FOXO4-DRI peptide mechanism of action.” 2025. (Peptide Gurus)
-
ParticlePeptides, “FOXO4-DRI: A Breakthrough Peptide Targeting Aging at Its Roots.” 2025. (Particle Peptides)
-
XcelPeptides, “FOXO4-DRI Peptide: A Key Player in Cellular Senescence Research.” 2024. (Xcel Peptides)
-
PeptideSciences blog, “Cellular Senescence: What Can Be Done About It?” (FOXO4-DRI category) 2025. (Peptide Sciences)
-
World-of-Peptides, “FOXO4-DRI Everything You Need to Know.” 2023. (World Of Peptides)
-
PeptidesCalculator, “FOXO4-DRI: Peptide for Cellular Senescence and Anti-Ageing Studies.” 2025. (Peptides Calculator)
-
BiotechPeptides, “Cell Aging Research and FOXO4-DRI Peptide.” 2022. (Biotech Peptides)
-
InnoPeptides, “FOXO4-DRI Peptide: A Breakthrough in Oncology and Cell Signalling Research.” 2025. (Innopeptides)


