Interleukin-16 (IL-16) and Cardiovascular Disease Risk: Evidence, Mechanisms, and Mitigation
Introduction
Interleukin‑16 (IL‑16) is a multifaceted cytokine with emerging relevance to cardiovascular health. Initially discovered as a lymphocyte chemoattractant, IL‑16 is now recognized for its broader roles in inflammation, immune signaling, and tissue remodeling.
With the SmartVascularDx PULS test including IL‑16 as one of its seven markers, understanding its implication for cardiovascular risk is increasingly important. This article delves into:
-
The biology of IL‑16 and its role in CVD.
-
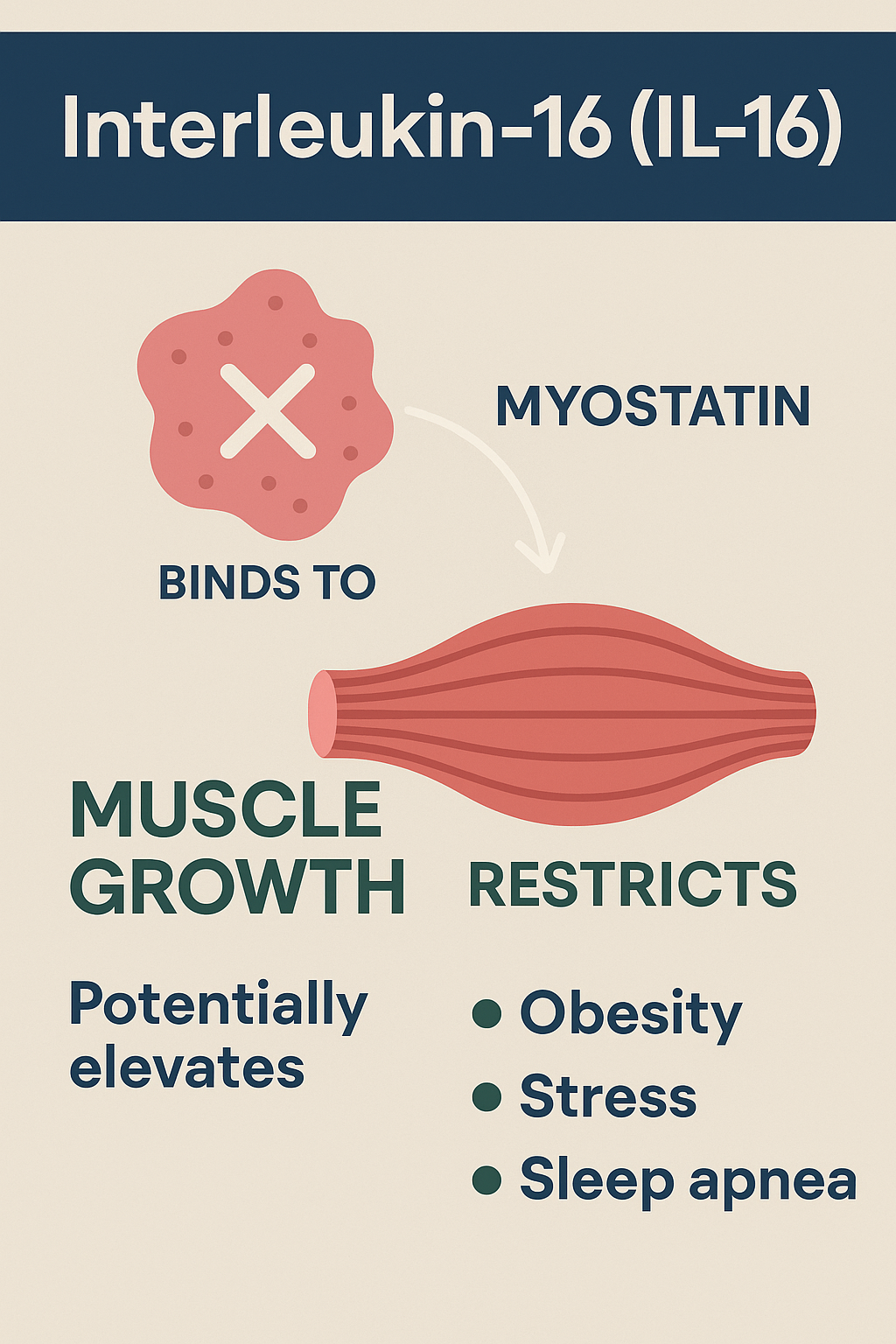
What factors elevate IL‑16—especially stress, obesity, and sleep apnea.
-
Evidence-based strategies for reducing IL‑16 levels.
-
Clinical insights and future directions.
Let’s explore the latest scientific insights.
1. Biology of IL-16
IL‑16 is produced as a precursor protein in many tissues and, via caspase‑3 cleaving, generates an active form that acts as a chemoattractant (binding receptors like CD4 and CD9) and regulates immune cell trafficking. It participates in inflammatory responses and is elevated during pyroptosis—a type of inflammatory cell death.
Recent proteomic studies have highlighted IL‑16 as a biomarker in inflammatory diseases such as lupus nephritis and skin lesions in lupus erythematosus, and as a potential drug target due to favorable profiles in animal models.
2. IL-16 and Cardiovascular Disease: A Complex Relationship
A. Potential Protective Roles
Some data suggest IL‑16 may play a protective role in atherosclerotic plaque stability. Elevated IL‑16 in carotid plaques has been associated with more stable plaque components (e.g., collagen, elastin, regulatory T cells), correlating with decreased postoperative cardiovascular events.
B. Potential Risk Associations
On the other hand, epidemiological findings are mixed. Elevated circulating IL‑16 in population studies has been associated with increased myocardial infarction risk, particularly in women. Among those with diabetes, individuals with higher IL‑16 and concurrent cardiovascular issues had ~50% higher IL‑16 compared to those without complications.
Additional studies show that IL‑16 may contribute to cardiac fibrosis, myocardial stiffness, and the production of pro-inflammatory cytokines such as IL‑1β and IL‑6, potentially increasing risk of acute cardiac events.
Summary: IL-16 may confer both protective and harmful effects depending on context—stabilizing advanced plaques in localized tissues, yet elevated systemic levels may signal heightened CVD risk.
3. What Elevates IL-16?
A. Obesity
Studies indicate that serum IL‑16 is higher in individuals with obesity than in normal-weight peers. Post-bariatric surgery, IL‑16 levels initially rise (6 months), then return toward baseline by 12 months.
Mechanistically, IL‑16 modulates adipogenesis, lipid handling, fibrosis, and inflammatory signaling in fat cells, suggesting it plays an active role in metabolic dysfunction.
B. Stress and Sleep Disruption
Specific data on IL‑16 in stress or sleep apnea are lacking. However:
-
Chronic stress elevates related pro-inflammatory cytokines, notably IL‑6 (which may act similarly to IL‑16 in some inflammatory pathways).
-
Sleep apnea, strongly tied to systemic inflammation and metabolic stress, increases markers like IL‑6 and CRP, and contributes to cardiovascular risk via stress hormones, oxidative stress, and endothelial dysfunction.
Given IL‑16 is upregulated in contexts of inflammation and cell stress, it is plausible—and supported indirectly—that stress, obesity, and sleep apnea may elevate IL‑16, though direct human studies linking sleep apnea or stress specifically to IL‑16 remain to be published.
C. Other Inflammation Pathways
As an inflammatory cytokine, IL‑16 may also rise in broader immune activation, autoimmune disease, infection, or localized tissue inflammation.
4. How to Address Elevated IL-16: Evidence-Based Strategies
Even without direct IL‑16–targeted therapies, you can address its upstream triggers. Here's what the literature suggests:
A. Weight Loss & Metabolic Improvement
Bariatric surgery led to normalization of IL‑16 levels by 12 months.
Therefore, dietary changes, increased physical activity, and metabolic risk reduction are likely to lower IL‑16 indirectly.
B. Sleep Apnea Treatment
While not studied for IL‑16 specifically, CPAP therapy clearly reduces systemic inflammation, improves blood pressure, reduces stress hormones, and reverses endothelial dysfunction. It likely influences inflammatory cytokines overall, including IL‑16.
C. Stress Reduction
Chronic stress management (e.g., mindfulness, meditation, therapy, exercise) has been shown to reduce inflammatory cytokines like IL‑6; by analogy, it may also help modulate IL‑16.
D. Anti-inflammatory Lifestyle and Therapies
-
A heart-healthy diet rich in anti-inflammatory nutrients likely reduces overall cytokine burden.
-
Statins, ACE inhibitors, and lifestyle modifications improve endothelial function and dampen inflammation broadly, potentially normalizing IL‑16-related pathways.
-
Emerging therapies targeting IL‑6 (e.g., canakinumab) provide proof of concept for cytokine-targeted cardiovascular risk reduction—suggesting that a future IL‑16-directed approach may be possible.
5. Clinical Implications & Future Directions
-
IL‑16 in PULS Testing: Elevated IL‑16 may indicate active systemic inflammation, metabolic stress, or early atherosclerotic processes—particularly in obese or sleep-disrupted individuals.
-
Context-Dependent Risk: If paired with imaging, high IL‑16 in plaques may signal stability—but high circulating IL‑16 suggests increased systemic risk, especially in women or patients with diabetes.
-
Personalized Intervention: Addressing obesity, sleep apnea, and chronic stress may offer the most effective means of reducing IL‑16 and associated CVD risk.
-
Research Needs: Direct clinical studies are needed to clarify:
-
IL‑16 levels in patients with sleep apnea or chronic stress.
-
The effect of interventions (e.g., CPAP, weight loss) on IL‑16.
-
Whether IL‑16 is a modifiable therapeutic target for CVD prevention.
-
Conclusion
Interleukin‑16 remains an intriguing, if complex, biomarker in cardiovascular health. It may reflect both protective plaque-related activity and systemic inflammatory risk, depending on context. Evidence supports that obesity, sleep apnea, and stress likely contribute to elevated IL‑16—though direct studies are still needed.
To mitigate elevated IL‑16 and potential cardiovascular risk, prioritizing weight management, sleep quality, and stress reduction, alongside traditional cardiovascular risk reduction strategies, is prudent. As research progresses, IL‑16 may emerge as both a diagnostic marker and therapeutic target.
References
-
Lund University dissertation: “Interleukin 16 in Atherosclerosis and Cardiovascular Disease,” showing IL‑16’s plaque-stabilizing roles and statistical links to MI risk and diabetes complications (Frontiers, sleep.theclinics.com, ScienceDirect, Lund University).
-
Stroke/AHA study: High IL‑16 in carotid plaques linked to decreased CVD events post-surgery, suggesting protective roles (American Heart Association Journals).
-
Frontiers in Endocrinology (2024): IL‑16 elevated in obesity, dynamic post-bariatric surgery, influences adipogenesis, fibrosis, inflammation (Frontiers).
-
Oncotarget review: IL‑16 associated with plaque stability but also cardiac fibrosis, stiffness, pro-inflammatory cytokine production, increased MI risk (Oncotarget).
-
Proteomics in Frontiers Immunology: IL‑16 biology, cleavage, pyroptosis linkage, and disease biomarker potential (Frontiers).
-
Johns Hopkins (2017): Sleep apnea increases stress hormones and cardiovascular stress, reversible with CPAP (The Hub).
-
MDPI International Journal of Molecular Sciences: Sleep apnea fuels systemic inflammation via IL‑6 & TNF-α pathways (MDPI).
-
Sleep clinics review: Sleep apnea, obesity, and inflammation contribute to CVD through multiple mechanisms (sleep.theclinics.com).
-
Social Stress entry (Wikipedia): Chronic social stress raises IL‑6 and broader inflammatory markers (Wikipedia).
-
Endothelial Dysfunction entry (Wikipedia): Inflammatory markers including IL‑6 linked to endothelial dysfunction—informative for systemic inflammation context (Wikipedia).
-
ASPC page: Inflammation and CVD risk via markers like IL‑6 and hs‑CRP (aspconline.org).
-
Lancet (IL‑6 inhibition study): Canakinumab reduces major CVD events via IL‑6 and hs‑CRP reduction—example of cytokine-targeted therapy (The Lancet).