SCARB1 (SR‑BI): When “Good Cholesterol” Isn’t Enough
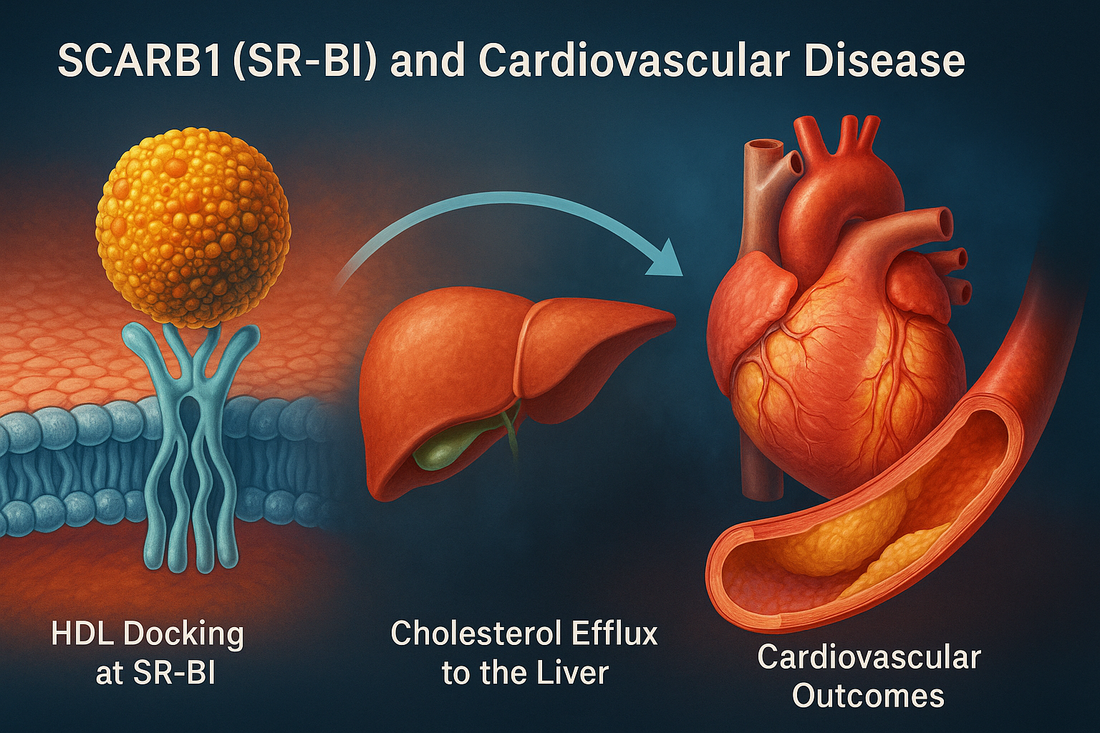
Most patients hear that high HDL cholesterol is protective. Yet cardiologists also see people with high HDL‑C who still develop coronary disease. A major reason for that paradox involves SCARB1, the gene that encodes SR‑BI (scavenger receptor class B type I)—the principal receptor that enables HDL to deliver cholesterol back to the liver for excretion. If SR‑BI does not work well, HDL particles can look plentiful on a blood test but fail to do their job.
Your CardiaX report may include one or more SCARB1 single‑nucleotide polymorphisms (SNPs)—commonly rs5888 and rs10846744—that influence receptor expression or function. This article explains what those results mean, how SCARB1 biology ties to cardiovascular disease (CVD), and which lifestyle, nutraceutical, peptide, and medication strategies best mitigate risk.
SCARB1 101: What SR‑BI Actually Does
-
Location & product. SCARB1 resides on chromosome 12q24.31 and encodes SR‑BI, a cell‑surface receptor expressed in the liver, steroidogenic tissues, macrophages, and endothelium.
-
Selective cholesteryl‑ester uptake. Unlike the LDL receptor, SR‑BI does not endocytose the entire HDL particle. Instead, it performs “selective uptake”—pulling cholesteryl esters out of HDL into hepatocytes while allowing the HDL particle to return to circulation and make another cholesterol pickup.
-
Reverse cholesterol transport (RCT). SR‑BI function is a rate‑limiting step in RCT—the “garbage truck” route that hauls cholesterol out of plaques and back to the liver for bile excretion.
-
Endothelial effects. HDL binding to SR‑BI triggers eNOS activation, anti‑inflammatory signaling, and antioxidant defenses in the vascular lining.
-
Steroidogenic support. In adrenal and gonadal tissues, SR‑BI provides cholesterol substrate for hormone synthesis.
Bottom line: SCARB1 is about HDL function, not just HDL quantity. When SR‑BI is impaired, HDL‑C may rise, but cholesterol efflux and vascular protection fall.
Key SCARB1 Variants You May See on CardiaX
-
rs5888 (c.1050C>T; A350A). A synonymous coding variant linked in several cohorts to altered SR‑BI expression, higher HDL‑C, and variable associations with CAD. Despite being “silent,” it can influence mRNA stability or be a marker for functional haplotypes.
-
rs10846744 (intron 1). Strongly studied in diverse populations; associated with carotid intima‑media thickness, coronary disease, and inflammatory profiles, likely via effects on regulatory motifs that control SCARB1 expression.
-
Loss‑of‑function mutations (rare). Individuals can present with very high HDL‑C yet increased coronary risk—a striking example that HDL quantity without function is insufficient.
Your report may annotate these with “risk,” “neutral,” or “protective” calls based on the scientific literature and internal validation. We integrate that call with phenotype (lipids, triglycerides, insulin resistance, inflammation, PULS biomarkers) to craft a plan.
How SCARB1 Variants Raise Cardiovascular Risk
-
Impaired reverse cholesterol transport
-
Reduced SR‑BI activity means less HDL‑mediated efflux from foam cells and slower hepatic clearance of cholesteryl esters. Plaques become lipid‑loaded and prone to inflammation.
-
-
HDL dysfunction despite normal or high HDL‑C
-
Standard lipid panels count how much cholesterol HDL carries, not whether HDL works. SCARB1 variants can yield “pretty” numbers with poor efflux capacity, misleading both patients and clinicians who track only HDL‑C.
-
-
Endothelial signaling deficit
-
SR‑BI is a mechanosensor and co‑receptor for HDL on endothelium. When signaling is blunted, eNOS activity falls, oxidative stress rises, and adhesion molecules increase—conditions favoring atherogenesis.
-
-
Pro‑inflammatory milieu
-
Some SCARB1 risk alleles correlate with higher hs‑CRP and IL‑6. Inflammation further impairs HDL remodeling, setting up a vicious cycle.
-
Phenotypic Patterns Suggestive of SCARB1‑Related Risk
-
HDL‑C in the high‑normal or elevated range with unexpected atherosclerosis (CAC, CIMT, or coronary events).
-
High triglycerides or insulin resistance, which degrade HDL quality and overwhelm the RCT system.
-
Elevated Lp(a) or small, dense LDL—particles that accelerate plaque formation when RCT is sluggish.
-
Endothelial dysfunction (e.g., elevated central BP, PULS injury biomarkers, microalbuminuria) despite “good” HDL‑C.
What Makes SCARB1 Risk Worse?
-
Smoking and environmental toxins (oxidize HDL and cripple SR‑BI signaling).
-
High refined‑carbohydrate and alcohol intake (raise triglycerides, generate small HDL, and impair efflux).
-
Visceral adiposity and inactivity (raise inflammatory cytokines and suppress HDL function).
-
Uncontrolled hypothyroidism (lowers LDL receptor activity and HDL turnover).
-
Chronic sleep restriction or OSA (increase oxidative stress and sympathetic tone).
The Intervention Playbook
Because SCARB1 is about function, our strategy emphasizes HDL quality, efflux, and inflammation control, not just “raising HDL‑C.”
1) Lifestyle Foundations
Nutrition (HDL‑function forward)
-
Mediterranean pattern: Vegetables, legumes, nuts, extra‑virgin olive oil, fatty fish. Improves HDL efflux capacity and lowers inflammation.
-
Protein at each meal to reduce hepatic VLDL output and keep triglycerides down.
-
Limit sugars, refined starches, and alcohol—the fastest way to degrade HDL functionality.
-
Add polyphenols (berries, olive‑oil phenolics, green tea, cocoa) that enhance HDL antioxidant capacity.
Exercise
-
150–300 minutes/week of aerobic work plus 2–3 resistance sessions improves HDL particle remodeling and reduces triglycerides.
-
Include zone‑2 for mitochondrial efficiency and intermittent HIIT to stimulate favorable lipoprotein enzymes (e.g., LPL).
Body composition & sleep
-
Target 7–10% fat loss if overweight to shrink VLDL production.
-
Prioritize 7–9 hours of sleep; evaluate and treat OSA.
2) Nutraceuticals (clinic‑guided)
-
Omega‑3 fatty acids — Omega 1300. Lowers triglycerides, reduces inflammation, and improves HDL particle function.
-
Coenzyme Q10 + omega‑3 — CoQ10 Omega. Supports endothelial mitochondria and improves flow‑mediated dilation.
-
Curcumin — Curcumin Complex. NF‑κB downregulation; improves HDL’s antioxidant properties.
-
Magnesium glycinate. Enhances insulin sensitivity and vascular tone.
-
Niacin (select patients). Can improve HDL function and lower Lp(a) modestly; outcome benefit is context‑dependent—best reserved for high‑risk profiles not at LDL targets.
-
Vitamin D and K2 as needed. Support vascular health and may modulate inflammation and calcification physiology.
We do not “chase HDL‑C.” The aim is HDL functionality and global risk reduction.
3) Peptides (physician‑supervised)
-
MOTS‑c. Enhances mitochondrial flexibility, insulin sensitivity, and exercise capacity—downstream benefits include lower triglycerides and improved HDL function.
-
KPV. Potent anti‑inflammatory support that can improve endothelial milieu when inflammatory biomarkers or PULS scores are elevated.
-
BPC‑157. Microvascular and endothelial repair; helpful when vascular injury or gut‑mediated inflammation contributes to risk.
Peptides are for research purposes or specific indications and used only under physician supervision consistent with our clinic policies.
4) Medications (evidence‑based anchors)
-
Get LDL‑C to goal—aggressively. With impaired RCT, apoB‑lowering is paramount.
-
High‑intensity statins are first‑line.
-
Ezetimibe adds ~15–20% LDL reduction.
-
PCSK9 inhibitors (or siRNA) for very high‑risk profiles, especially with premature CAD or high Lp(a).
-
-
Triglyceride control. If TG ≥150–200 mg/dL, address lifestyle first; consider icosapent ethyl or fenofibrate (particularly in high‑TG, low‑HDL patterns).
-
Blood pressure: Favor ARBs/ACEi for vascular protection; add MRAs in resistant HTN.
-
Antithrombotic therapy: Individualize based on ASCVD status; SCARB1 itself does not dictate antiplatelet use.
Putting It All Together: A Practical Protocol
-
Assess phenotype
-
Lipids with apoB and Lp(a), fasting TG, hs‑CRP, insulin resistance markers.
-
Structural/functional tests as indicated: CAC, CIMT, central BP, PULS.
-
-
Personalized plan
-
Nutrition: Mediterranean, low‑sugar, alcohol‑light; protein and fiber targets.
-
Exercise: Zone‑2 base + resistance; add intervals 1–2× weekly.
-
Supplements: Omega 1300, CoQ10 Omega, Curcumin Complex, magnesium; consider niacin selectively.
-
Peptides: MOTS‑c and KPV when inflammation or metabolic inflexibility persists.
-
Medications: Statin ± ezetimibe/PCSK9 to reach apoB <65–80 mg/dL depending on risk; triglyceride‑focused therapy if needed; BP optimization.
-
-
Track and adapt
-
Recheck labs and vascular metrics at 3–6 months; evaluate symptoms and fitness.
-
Do not rely on HDL‑C alone; if available, consider HDL efflux or oxidized HDL assays for deeper insight.
-
Case Vignette
Patient: 54‑year‑old woman, CardiaX shows SCARB1 rs10846744 risk allele. HDL‑C 76 mg/dL, LDL‑C 118 mg/dL, TG 190 mg/dL, apoB 100 mg/dL. Father had MI at 58. CAC score 110. She exercises sporadically and drinks 2–3 glasses of wine on most evenings.
Plan:
-
Nutrition: Mediterranean pattern, alcohol ≤3/week, fiber ≥30 g/day, protein 1.2 g/kg/day, added polyphenols (berries, EVOO).
-
Exercise: 4 days/week zone‑2 (45–60 min) + 2 resistance days.
-
Supplements: Omega 1300, CoQ10 Omega, Curcumin Complex, magnesium.
-
Peptide: MOTS‑c for 4 weeks to improve metabolic flexibility; KPV short course for inflammation.
-
Medications: High‑intensity statin + ezetimibe to target apoB <65 mg/dL; icosapent ethyl initiated for TG control.
-
Follow‑up: At 6 months, apoB 58 mg/dL, TG 118 mg/dL, hs‑CRP 0.7 mg/L; exercise capacity improved; repeat central BP lower. Despite HDL‑C now 63 mg/dL (lower than before), her overall risk is meaningfully reduced because transport function and apoB burden improved.
FAQs
Is a high HDL‑C level always good in SCARB1 carriers?
No. With impaired SR‑BI, HDL‑C can be misleadingly high. Focus on apoB, triglycerides, inflammation, and clinical imaging rather than chasing HDL‑C.
Should I take a drug that raises HDL‑C if my SCARB1 variant is risky?
Not usually. Trials that simply raise HDL‑C without improving efflux/apoB have not reduced events. The priority is apoB lowering, triglyceride control, and inflammation reduction.
How does SCARB1 differ from CETP genes?
CETP moves cholesteryl esters between lipoproteins; SCARB1 moves cholesterol from HDL into cells (especially hepatocytes). Both influence HDL metrics, but SCARB1 is the “handoff to the liver.”
The Bottom Line
SCARB1 variants teach an important lesson: HDL function beats HDL number. When SR‑BI is suboptimal, reverse cholesterol transport slows, endothelial signaling falters, and plaque risk rises—even if HDL‑C looks impressive. CardiaX helps surface that hidden risk so we can act early.
A high‑impact plan blends Mediterranean nutrition, structured exercise, triglyceride control, inflammation reduction, and aggressive apoB lowering, supported by targeted nutraceuticals—and, in select cases, physician‑supervised peptides to optimize metabolic and endothelial health.
References
-
Acton S, et al. Identification of scavenger receptor SR‑BI as a high density lipoprotein receptor. Science. 1996.
-
Zanoni P, et al. Rare variant in SCARB1 raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016.
-
Vergeer M, et al. High‑density lipoprotein and apoA‑I: the role of HDL function in cardiovascular disease. Nat Rev Cardiol. 2010.
-
Teslovich TM, et al. Biological, clinical and population relevance of 95 loci for blood lipids (includes SCARB1). Nature. 2010.
-
Naj AC, et al. Association of SCARB1 intronic variant rs10846744 with subclinical atherosclerosis. Circ Cardiovasc Genet. 2010.
-
Besler C, et al. Mechanisms underlying adverse effects of HDL on eNOS in the presence of inflammation. J Clin Invest. 2011.